The periodic table is a remarkable framework that organizes all known chemical elements based on their atomic number, electron configurations, and recurring chemical properties. Often referred to as the “chemist’s bible,” this table is an indispensable tool for scientists, researchers, and students alike.
From predicting chemical behaviors to explaining the properties of elements, the Periodic Table embodies simplicity and brilliance in scientific design.
Its layout may appear straightforward, but the underlying principles reveal the profound intricacies of matter itself.
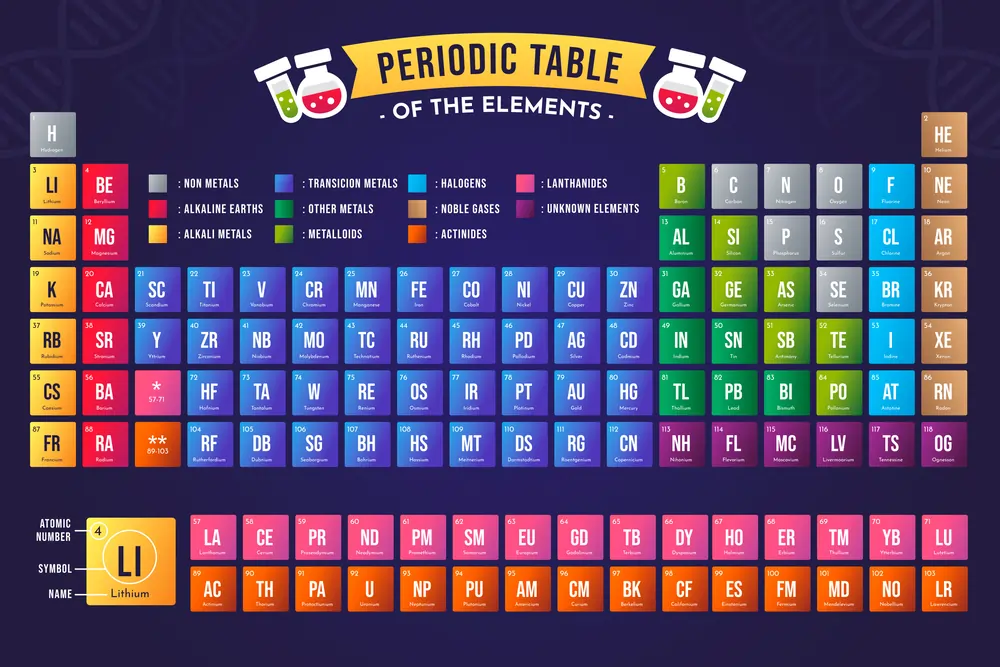
Image source: Freepik.com
History of the Periodic Table
Early Attempts to Classify Elements
Before the modern table emerged, scientists struggled to classify elements systematically. Johann Wolfgang Döbereiner’s triads grouped elements with similar properties in threes. Later, John Newlands proposed the “Law of Octaves,” suggesting that elements repeated properties every eighth place—though his theory had limitations.
Dmitri Mendeleev’s Groundbreaking Contribution
The Periodic Table as we know it began with Dmitri Mendeleev in 1869. He arranged elements by atomic weight and left gaps for undiscovered elements, accurately predicting their properties. This foresight solidified his contribution as monumental in chemistry.
Evolution into the Modern Periodic Table
With advancements in atomic theory, Henry Moseley revised the table by organizing elements by atomic number instead of weight. This corrected inconsistencies and reinforced the predictive power of the Periodic Table.
Structure of the Periodic Table
Periods and Groups Explained
The table is divided into horizontal periods and vertical groups. Periods represent elements with the same number of electron shells, while groups contain elements with similar valence electron configurations and chemical properties.
Metals, Nonmetals, and Metalloids
Elements are broadly categorized into metals, known for their conductivity and malleability, nonmetals, insulators and brittle, and metalloids, which exhibit both properties.
Trends in the Periodic Table
- Atomic Radius: Decreases across a period and increases down a group.
- Ionization Energy: Increases across a period and decreases down a group.
- Electronegativity: Follows a similar trend as ionization energy, with fluorine being the most electronegative element.
Periodic Law
The Periodic Law states that the properties of elements are a periodic function of their atomic numbers. This law underpins the table’s design, explaining why elements in the same group exhibit comparable properties. For instance, alkali metals like sodium and potassium react vigorously with water, a pattern predicted by the Periodic Law.
Types of Elements in the Table
Alkali Metals
Group 1 elements are highly reactive metals like lithium and sodium. Their reactivity increases down the group.
Alkaline Earth Metals
Group 2 elements like magnesium and calcium are less reactive but essential for life and industrial applications.
Transition Metals
Located in the middle of the table, these elements, including iron and gold, are known for their strength, conductivity, and vibrant compounds.
The Periodic Table simplifies complex chemistry by providing a visual representation of elemental relationships. Beyond academia, it has practical applications in medicine, agriculture, and technology. From diagnosing diseases with isotopes to advancing battery technologies, the table’s relevance extends far beyond labs.
Halogens and Noble Gases
Halogens (Group 17) are reactive nonmetals, while noble gases (Group 18) are inert due to their complete valence electron shells.

